Generally speaking
Two kinds of local analysis are possible, one invoked by the program dec.py, the other by amplian.py. We will define these two as shotgun mode (albeit improperly) and amplicon mode. The input is always a sorted bam file. The window size should be slightly smaller than the read length.
Shotgun mode: region longer than the reads
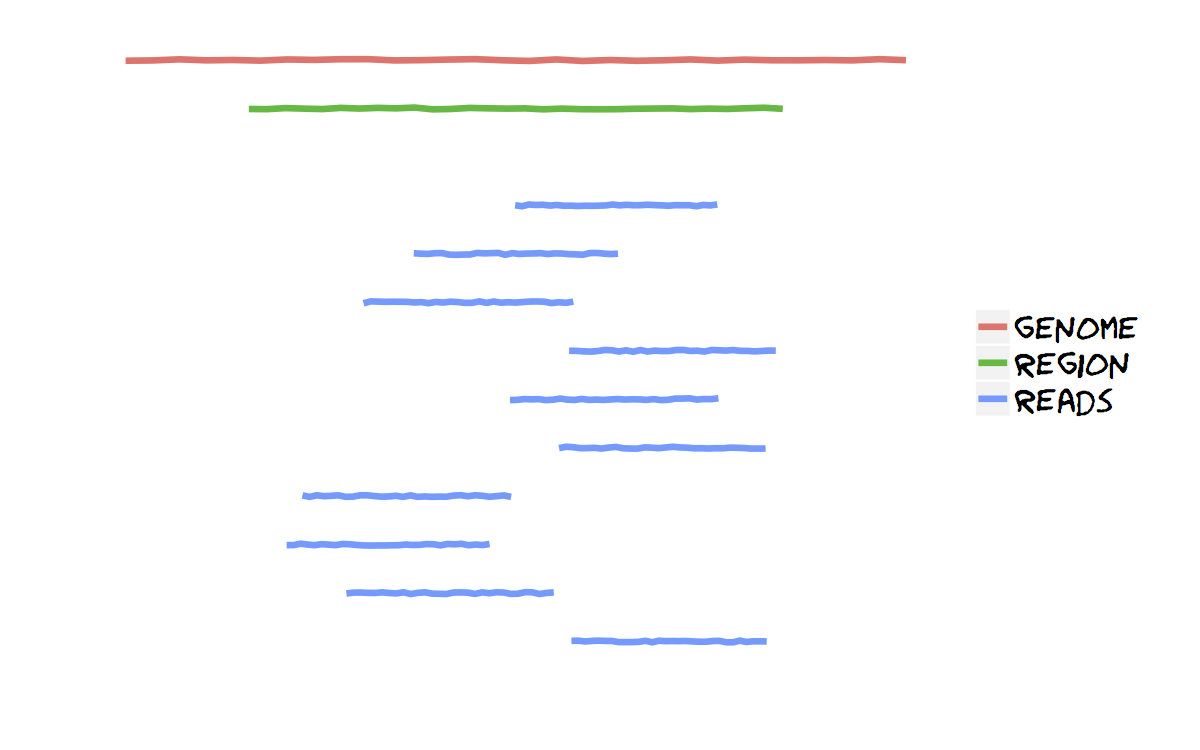 This is performed when the amplicon is longer than the typical read length and we don’t want to focus our analysis on a specific region of this amplicon.
This is performed when the amplicon is longer than the typical read length and we don’t want to focus our analysis on a specific region of this amplicon. dec.py cuts on the aligment a set of overlapping regions (parameters can be adjusted, run dec.py -h for help), and passes them to diri_sampler for error correction and local haplotype reconstruction. This is the first step of the global analysis. Additionally, it runs snv.py for SNV calling.
Amplicon mode: region covered by a single read
![]() When the amplicon size is smaller than the read length or comparable to it, it is in general more appropriate to use
When the amplicon size is smaller than the read length or comparable to it, it is in general more appropriate to use amplian.py, which extracts the multiple alignment corresponding to the specified amplicon. By default, this includes reads that cover at least 95% of the amplicon region (this can be adjusted with the option -m MIN_OVERLAP). It then runs diri_sampler for error correction and snv.py for SNV calling.
Examples
Shotgun mode: dec.py
Run dec.py -h for help.
[user@host]$ dec.py -h
Usage: dec.py [options]
Options:
-h, --help show this help message and exit
Input files:
Required input
-b IN_BAM, --bam=IN_BAM
file with aligned reads in .bam format
-f IN_FASTA, --fasta=IN_FASTA
reference genome in fasta format
. . . more options . . .You should specify the window size (default is 201). Intermediate files are now kept by default. Next line runs with a window length of 300 and keeps intermediate files. ref_file.fasta contains the reference sequence that has been used to align reads.
[user@host]$ dec.py -b my_sorted_bam.bam -f ref_file.fasta -w 300 -kAmplicon mode: amplian.py
As usual, the help is available with -h.
[user@host]$ amplian.py -b my_sorted_bam.bam -f amplicon.fasta -m 0.95amplian.py uses the option -m to specify minimum overlap that the reads are required to have with the amplicon in order to be included (default 95%). The file amplicon.fasta contains the reference sequence used to align reads. If this is longer than the read length, the user can specify a shorter region with the option -r chromosome_name:region_start-region_stop.
Find the highest diversity region
Starting from version 0.8, amplian is capable of detecting the region corresponding to the highest Shannon entropy. The size of the region is the trimmed mean of the read length (_i.e._ the mean computed on reads between 10th and 90th percentile of length). This method can be used, for example, if one is interested in the lower bound on the number of haplotypes. See also our paper on PLoS ONE (figure 1). If matplotlib is installed, a plot of the entropy is saved in file entropy.pdf. All entropy values are saved in the comma-separated value file entropy.csv.
Output of local analysis
Shotgun mode
Suppose we are analyzing a region of 1500 bp, and we chose window size of 300. By default, windows overlap such that (except for the boundaries of the region) each base is covered by three windows (option -s). So dec.py will produce windows 1-300, 101-400, 201-500, 301-600 and so on. All these are passed to diri_sampler and local haplotype are inferred. For each window there will be several files produced. We describe here two of these, the support and the sampling file.
The support file has the support.fas suffix. It is a fasta file with the reconstructed haplotypes. The header of each sequence contains information on the quality of the reconstruction (posterior probability of the haplotype) and the frequency of the haplotype. For example,
>hap_0|posterior=1 ave_reads=235.168
CCTCAGATCACTCTTTGGCAACGACCCTTCGTCindicates that the reconstructed haplotype has a posterior probability of 1 (it was always present in the sampled Markov chain) and on average 235 reads were assigned to it. Good results have been obtained by discarding haplotypes with less than 90-95% support.
snv.py takes care of parsing the single nucleotide variants, check the strand bias and write a CSV file with all the information (suffix final.csv) in the directory snv.
Amplicon mode
amplian.py will analyse a single window, either passed by the user or determined by the program (see above). Consequently, the output files from the support to the SNV file will refer to this single window (see shotgun mode above).
Go back home